
Getting enough people to enroll and participate to the end of a clinical study is a major challenge. Most studies fail to enroll enough subjects to even begin. And if you can’t enroll enough people, you certainly can’t gather enough data to ensure statistically sound results.
Today, researchers are addressing these challenges by decentralizing studies – offering components of the study to the patient at home, instead of requiring participants to visit study sites. As of 2021, 48%-95% of research sites report using at least one form of decentralized technology.1
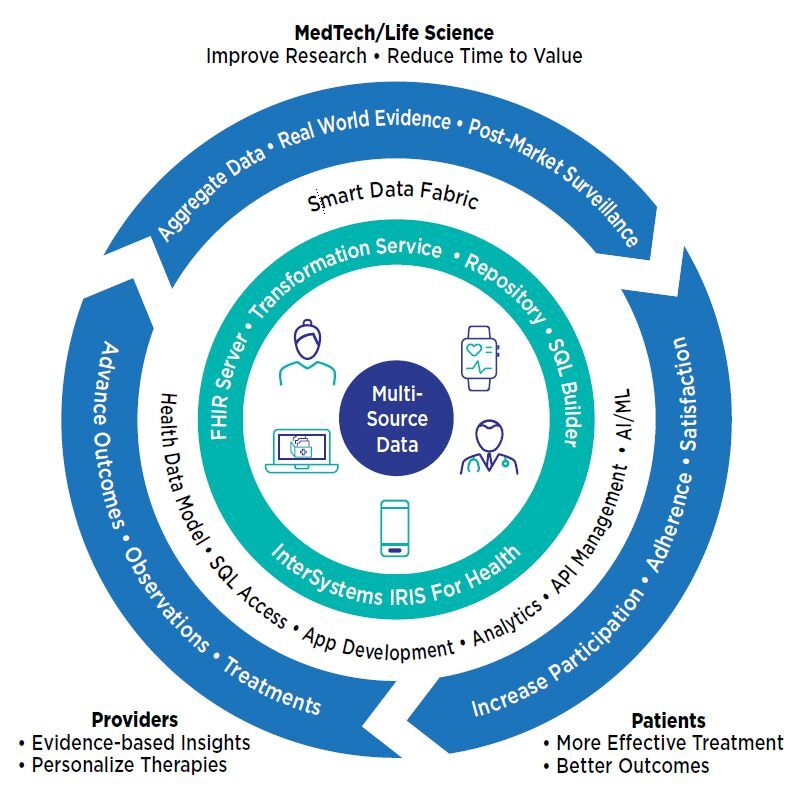
InterSystems IRIS for Health™ data platform supports decentralized and hybrid study models, enabling researchers to gather data from study participants, connected remote, wearable, or implanted medical devices, and electronic health records, health information exchanges, and other sources. Combined and transformed by InterSystems IRIS for Health into a single consistent format, such as HL7® FHIR® (FHIR), this data becomes the foundation for analysis and a comprehensive perspective on patient health and treatment effectiveness. Researchers in pharma, life sciences, and MedTech organizations can create a virtuous circle of feedback that drives continuous improvements in research, care, and outcomes.
Customer Use Case: Medical Device Maker
A major medical device maker conducted a decentralized study supported by InterSystems IRIS for Health data platform to gain a full, multi-dimensional view of therapy effectiveness. InterSystems IRIS for Health aggregated and transformed device, performance, time series, patient reported outcomes, and electronic health record data into FHIR format, and prepared it for access through the SQL already familiar to data scientists. Using InterSystems IRIS for Health, the data scientists gained the context they needed around the raw device data to answer critical questions using predictive and operational analytics. The platform and smart consumer technology enabled this customer to:
- Conduct a decentralized clinical study efficiently to validate and improve device performance
- Illuminate factors influencing variations in disease course among patients
- Provide guidance to clinicians on personalizing patient treatment
- Increase the value of the patient monitoring program
InterSystems IRIS for Health
InterSystems IRIS for Health can make studies more diverse and easier to participate in by gathering data from the subject at home, as well as devices and the hospital or clinic. By aggregating and normalizing diverse data types for analytics, machine learning, and AI systems, it helps researchers answer questions about treatment performance and function, track patient progress, and identify study issues before they become problems.
1 - McKinsey and Company, “No place like home? Stepping up the decentralization of clinical trials.”
https://www.mckinsey.com/industries/life-sciences/our-insights/no-place-like-home-stepping-up-the-decentralization-of-clinical-trials

























