
Conseguir que un número suficiente de personas se inscriban y participen hasta el final en un ensayo clínico es un reto importante. La mayoría de los ensayos no consiguen registrar a suficientes sujetos para siquiera empezar. Y si no se puede inscribir a un número suficiente de personas, ciertamente no se pueden recopilar datos suficientes para garantizar resultados estadísticamente sólidos.
Hoy en día, los investigadores están abordando estos retos mediante la descentralización de los ensayos ofreciendo componentes de los mismos al paciente en su casa, en lugar de requerir que los participantes visiten los centros donde se realiza. A partir de 2021, entre el 48 % y el 95 % de los centros de investigación afirman utilizar, al menos, una forma de tecnología descentralizada.1
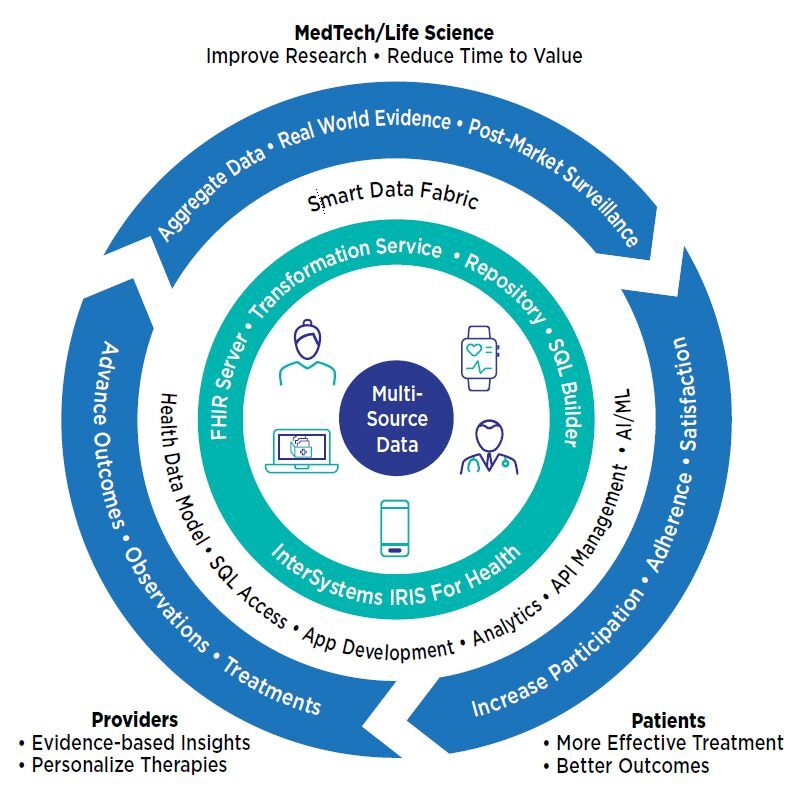
La plataforma de datos InterSystems IRIS for Health™ admite modelos de ensayos clínicos descentralizados e híbridos, facilitando que los investigadores recopilen datos de los participantes, de dispositivos médicos remotos, portátiles o implantados, así como de historias clínicas electrónicas, intercambios de información sanitaria y otras fuentes. Combinados y transformados por InterSystems IRIS for Health en un único formato coherente, como HL7® FHIR®, estos datos se convierten en la base del análisis y de una perspectiva global sobre la salud del paciente y la eficacia del tratamiento. Los investigadores de organizaciones farmacéuticas, de ciencias de la vida y de tecnología médica pueden crear un círculo virtuoso de retroalimentación que impulse mejoras continuas en la investigación, la atención y los resultados.
Caso práctico: fabricante de dispositivos médicos
Un importante fabricante de dispositivos médicos llevó a cabo un ensayo clínico descentralizado, con el apoyo de IRIS for Health, para obtener una visión completa y multidimensional de la eficacia de la terapia. InterSystems IRIS for Health agregó y transformó datos de dispositivos, rendimiento, series temporales, resultados comunicados por los pacientes e historias clínicas electrónicas en formato FHIR, y los preparó para el acceso a través de SQL, ya familiar para los científicos de datos. Gracias a ello, obtuvieron el contexto que necesitaban en torno a los datos brutos de los dispositivos para responder a preguntas críticas mediante análisis predictivos y operativos. La plataforma y la tecnología inteligente para el usuario facilitaron que la organización:
- Llevara a cabo un ensayo clínico descentralizado de manera eficiente para validar y mejorar el rendimiento del dispositivo.
- Aclarara los factores que influyen en las variaciones de la evolución de la enfermedad entre los pacientes.
- Orientara a los médicos sobre la personalización del tratamiento de los pacientes.
- Aumentars el valor del programa de seguimiento de pacientes.
InterSystems IRIS for Health
InterSystems IRIS for Health puede hacer que los ensayos clínicos sean más variados y que resulte más fácil participar en ellos, al recopilar datos del sujeto en casa, así como de los dispositivos y del hospital o la clínica. Al agregar y normalizar diversos tipos de datos para los sistemas de análisis, machine learning e IA, ayuda a los investigadores a responder preguntas sobre el rendimiento y la función del tratamiento, realizar un seguimiento del progreso de los pacientes e identificar cuestiones del estudio antes de que se conviertan en problemas.
1 - McKinsey and Company, ¿No hay lugar como el hogar? Aumentar la descentralización de los ensayos clínicos.
https://www.mckinsey.com/industries/life-sciences/our-insights/no-place-like-home-stepping-up-the-decentralization-of-clinical-trials

























